Sectors of activities
The Company manufactures drugs used in clinical oncology, cardiology, neurology and infectious/inflammatory diseases. Nucleis offers generic radiopharmaceutical drugs and aim to constantly develop new diagnostic tools and support innovative therapies.
Glucotrace
Tracer used in diagnostics and follow-up treatment in oncology, cardiology, neurology and infectious and inflammatory diseases.
Marketing authorization: MA Holder and direct sales in five countries: Belgium, the Netherlands, France, Luxembourg, Germany.

Authorised manufacturer of Blue Earth Diagnostics for AxuminTM and Authorised manufacturer of GE Healthcare for VizamylTM
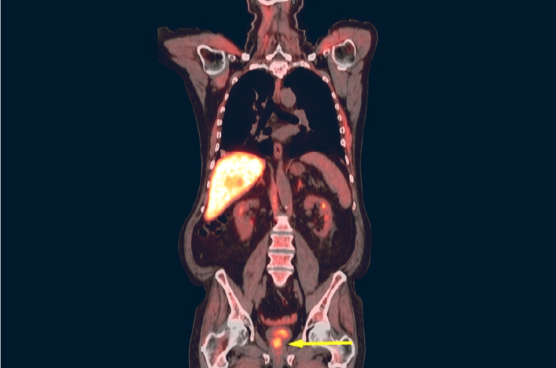
AxuminTMTM
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
More info: www.ema.europa.eu
www.blueearthdiagnostics.com/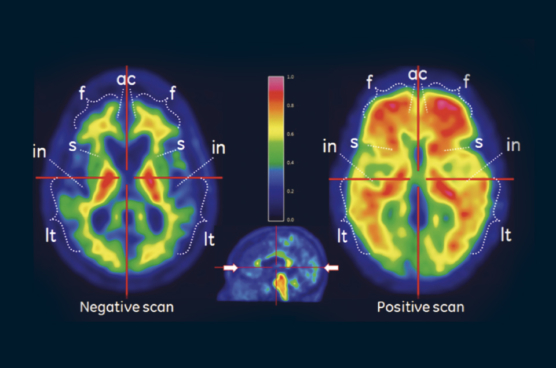
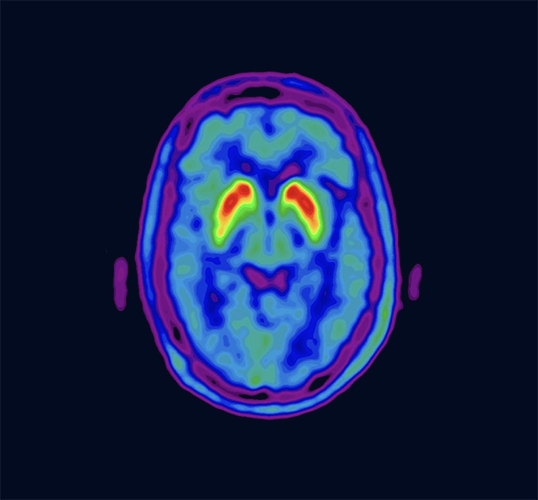
VizamylTMTM
Vizamyl is indicated for positron-emission tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive decline.
More info: www.ema.europa.eu
www.gehealthcare.comCertifications
Nucleis holds the following GMP certifications and manufacturing authorisations from the Belgian Federal Agency for Medicines and Health Products (FAMHP). Furthermore, Nucleis is the Marketing Authorization Holder (MAH) and manufacturer of Glucotrace (fludeoxyglucose (18F)) granted in Belgium, The Netherlands, Luxemburg, France and Germany.
GMP Certificate n° BE/GMP/2018/061 for Human Investigational Medicinal Products
MIA 1932 H
GMP Certificate n° BE/GMP/2018/060 for Human Medicinal Products
MIA 1932 IMP


